Data sources and population
Our population-based cohort study included all individuals aged 65 years and older who were enrolled in the Québec Integrated Chronic Disease Surveillance System (QICDSS) on April 1, 2019 (cohort enrollment date) for one year. Followed up. QICDSS links provincial health service administrative data since 1996 using unique patient IDs. [13]. Data include demographic, death registration, physician claims, and drug claim records obtained from the Provincial Health Insurance Board (Régie de l'assurance maladie du Québec). [RAMQ]) also has a hospital discharge abstract record (MED-ECHO) owned by the Quebec Ministry of Health and stored in RAMQ.Demographic data include place of residence, age, gender, and neighborhood-level social and material deprivation quintiles [14]. Starting January 1, 2019, physician claims will be coded using the International Classification of Diseases, Ninth Edition, Québec Adaptation (ICD-9-QC) and the ICD Tenth Edition Canadian Coding Standard (ICD-10-CA). Contains the diagnosis made. In the discharge record, the admitting diagnosis, primary diagnosis and up to 29 secondary diagnoses are coded using the ICD-9-QC system until March 31, 2006, and thereafter his ICD-10- Coded using CA system. Because Quebec has a universal healthcare system, QICDSS contains medical records for over 99% of the population. Additionally, drug insurance is mandatory in Quebec. Everyone aged 65 and over is covered by a public drug plan. However, about 10% are not covered because they want to maintain a private insurance plan or their medication is provided by the nursing home where they live.
Multimorbidity measures
We considered three widely used criteria to define multimorbidity: MM2+, MM3+, and MM4+. [3]. He also identified three lists of medical conditions that are commonly used in constructing complex disease measures. These lists were considered representative of the diverse medical conditions included in the complex disease measure based on health administrative data. [5] (List of diseases and ICD codes for each list are available in Supplemental Digital Content) [SDC] 01: Tables A1.1 to A1.4). First, the “Inclusive List” (L60) included all ICD codes corresponding to chronic diseases grouped into 60 diseases by a multidisciplinary team. [15]. This list was deemed to be of high quality in a previous systematic review as it met 6 of the 8 quality criteria used to define a robust multimorbidity control methodology. [5]. Second, the “core list” (L20) included his minimal core of 20 diseases identified in a systematic review by Ho et al. [3]. This minimal set of core diseases includes the chronic diseases that result in the highest disability-adjusted life years (DALYs) or years of life lost (YLLs) according to the Global Burden of Disease Project. [16]. We added osteoporosis to this list because it has been reported as one of the top 20 diseases that have the greatest impact on his DALYs in Canada. [17]. Third, the “Charlson & Elixhauser list” (L31) includes 31 diseases from a composite comorbidity index that combines both Charlson and Elixhauser comorbidity indices. [11, 12].
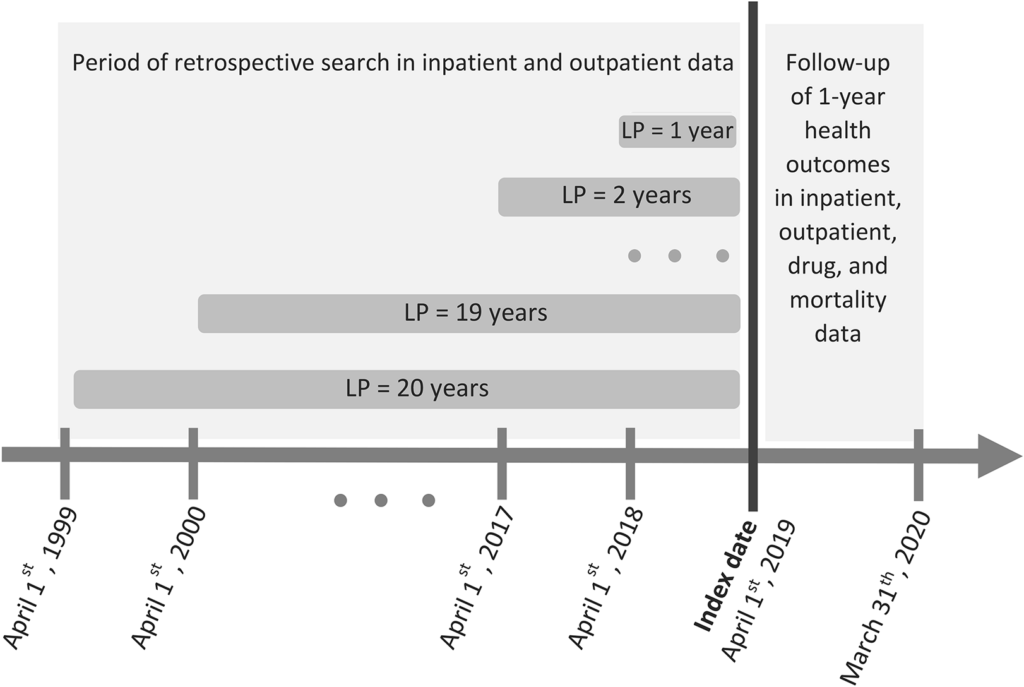
We employed different LPs ranging from 1 to 20 years to estimate multimorbidity prevalence at the date of cohort enrollment (April 1, 2019). We retrospectively searched her ICD diagnosis codes for each person and condition from hospitalization records and physician records up to April 1, 1999 (Figure 1). The selection of the 20-year LP was based on data availability in QICDSS, limiting the analysis to this period. We used the algorithm proposed by Klabunde et al. [18] To identify each disease in the administrative database, we searched both inpatient and outpatient records and determined whether (1) at least one diagnosis code (primary or secondary) was recorded in the admission record; 2) We identified an individual as having a disease if at least one diagnosis code was recorded. Two diagnosis codes were recorded on inpatient or outpatient physician claims within 2 years and at least 30 days apart.
Figure 1
Illustration of assessment of multimorbidity prevalence at reference date using various look-back periods (LPs) and 1-year health outcome measures. For example, if a person is 66 years old on April 1, 2019, a retrospective search in both inpatient and outpatient databases using 1-year LP would be from April 1, 2018 to March 2019. Runs until the 31st of the month. With a 20-year LP, the search is extended. From April 1, 1999 to March 31, 2019
result
We calculated that each measure of multimorbidity, calculated on April 1, 2019, was associated with multimorbidity and measured in the QICDSS during the 1-year follow-up (until March 31, 2020). We investigated its ability to predict six possible health outcomes. – Causes death, polypharmacy, hospitalization, and frequent visits to emergency departments (EDs), general practitioners (GPs), and specialist specialists (SPs). We defined polypharmacy as him claiming more than 10 different drugs in the follow-up year. We used a common unit (each active ingredient or combination has a separate common unit code) to identify each drug being claimed. These claims included medicines for acute and chronic diseases. We defined frequent ED visits using a commonly used threshold of 3 or more visits per year of follow-up. [19]. A visit to the ED is defined as one or more ED-related claims for up to two consecutive days. [20]. Frequent visits to the GP (7 or more visits) or SP (10 or more visits) in the follow-up year were defined using the 95th percentile of the annual number of visits to the ED in the Quebec adult population . [21, 22].
statistical analysis
We estimated the prevalence of multimorbidity for each criterion used to define multimorbidity, for each disease list, and for each LP (1–20 years), and estimated the prevalence of multimorbidity for each additional retrospective year. We calculated relative changes in disease prevalence (Figure 1).
We then used a logistic regression model to assess the impact of each criterion used to define multimorbidity on health outcomes. First, he built one baseline model for each health outcome. In this model, health outcomes were the dependent variables and covariates (age group, gender, material and social deprivation) were the predictor variables. For each combination of criteria used to define multimorbidity, to estimate the performance of multimorbidity indicators in predicting each health outcome beyond the results of baseline covariates and assess the impact of LP on predictive performance. We built 1,080 logistic regression models for (3 criteria), disease list (3 lists), LP (20 periods), and health outcomes (6 outcomes). Of note, analyzes of polypharmacy and medical service outcomes (hospitalization, ED, GP, SP visits) included only patients who were alive and on drug regimen throughout the 1-year follow-up. was. The performance of each model was evaluated using three measures: (1) The discriminative power of each model, i.e., the ability to use the c statistic (also known as the area under receiver operation) to accurately identify patients with an outcome within 1 year. characteristic curve) [23] (The difference in c statistics is excellent. [0.010] This value was considered important because covariates contributing to such differences may reduce confounding bias in observational studies. [24]); (2) the overall performance of the model calculated using the scaled Brier score. Values range from 0 to 1 (higher values indicate better performance). (3) the level of agreement between the observed and predicted probabilities of the outcome using the calibration intercept and slope; Values closer to zero and 1 indicate better predictions, respectively. [23].
All analyzes were performed using SAS 9.4 (SAS Institute, Cary, NC).
Supplementary and sensitivity analyzes
Considering the perceived variation in claims history, mortality risk, and health care resource utilization related to age and gender, we conducted a stratified analysis to estimate predictive performance according to these factors. We categorized the age group into 66-79 years old and her age 80 years or older, and also considered gender as a stratification factor. This approach allows us to investigate internal validity by evaluating the heterogeneity of performance between these groups, and given the large sample size and low model complexity, we can evaluate the average performance. is preferred over other approaches (such as bootstrapping). [25].
We also repeated all analyzes using disease-specific algorithms to take into account the reduced duration of chronicity for some diseases. Such algorithms have only been proposed in the literature for all diseases included in the “Core List” (L20), so we used them only for this list. These algorithms are described in the supplementary material (SDC01: Table A1.3). For the “Comprehensive List” (L60) and the “Charlson & Erikhauser List” (L31), these two lists limit the duration of LP to five years for all mental health disorders that undergo a course of remission or relapse. Did. [26, 27]. For the “core list” (L20), since hypertension is included in most of the multimorbidity measures, we added one supplementary disease (hypertension) to the list and reran all analyzes. [3].